Background: del(17p) is an independent prognostic marker associated with poor clinical outcome identified in a number of studies and has been incorporated into risk stratification models for multiple myeloma (Palumbo A et al J Clin Oncol 2015). Recent studies have reported that the percentage of myeloma cells with del(17p) by fluorescent in situ hybridization (FISH) can have a significant impact on outcomes (Corre J et al. Blood 2021; Thakurta A et al. Blood 2019; Thanendrarajan S et al. Haematologica 2017). Different collaborative groups have recommended different cut-points for the presence of del(17p) with clinical significance ranging from ≥20% or >55% of affected cells. Herein, we examined clinical outcomes of newly diagnosed multiple myeloma (NDMM) patients with del(17p) treated with novel combination triplet and quadruplet induction regimens, including bortezomib, lenalidomide, dexamethasone (VRd), carfilzomib, lenalidomide, dexamethasone (KRd), +/- daratumumab (DVRd and DKRd).
Methods: We conducted a chart review study of 578 NDMM patients treated with VRd, KRd, DVRd and DKRd at Memorial Sloan Kettering (MSK) from 1/1/2015 to 5/1/23. Cutoff date for analysis was 7/19/23. Only patients with available cytogenetic results (FISH and/or SNP-array) were included. Patients who received ≤1 prior cycle of another regimen for MM were included. We analyzed the percentage of cells with del(17p) by inter-phase FISH analyses and/or SNP-array with CD138 enrichment when FISH was not available. A cut-point of 20% for del(17p) was used (del(17p) <20% vs del(17p) ≥20%) and compared with patients with standard-risk disease. Discrete patient characteristics were summarized by frequency (percentage) and continuous characteristics were summarized by median (Interquartile Range, IQR). PFS and OS were evaluated by Kaplan-Meier method.
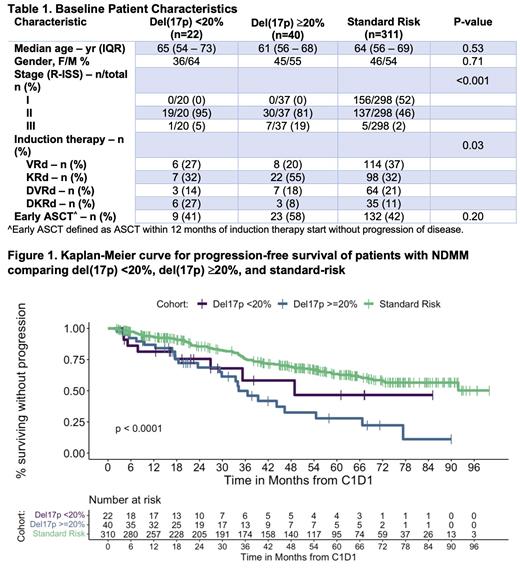
Results: There were 391 patients treated with triplet combination, of which 46 patients had del(17p) on baseline bone marrow biopsy. Among these patients, the presence of del(17p) in <20% of cells was detected in 13 patients, and the presence of del(17p) in ≥20% of affected cells was detected in 30 patients. Three patients did not have measurements. There were 187 patients treated with quadruplet combination, of which 20 had del(17p). Among these patients, 9 had del(17p) <20% of cells, 10 had del(17p) in ≥20% of cells, and one patient did not have measurements. There were 212 standard-risk NDMM patients treated with triplet induction and 99 patients treated with quadruplet regimens. The median age was 65 (IQR 54-73) for patients with del(17p) <20%, 61 (IQR 56-68) for del(17p) ≥20%, and 64 (IQR 56-69) for standard-risk patients (P=0.53). Other patient's baseline characteristics are summarized in Table 1.
Best ORR among the three groups after triplet or quadruplet induction regimens were high: 95% for patients with del(17p) <20%, 93% for patients with del(17p) ≥20%, and 90% for standard-risk patients (P=0.53). After a median follow-up of 56.9 months, 2-year estimated PFS was 76% (95%CI, 59%-97%), 69% (95%CI, 55%-86%), and 85% (95%CI, 81%-90%) for del(17p) <20%, del(17p) ≥20%, and standard-risk groups, respectively. Median PFS was 48.9 months (95%CI, 26.9-not reached [NR]) for patients with del(17p) <20%, 34.3 months (29.8-66.7) for del(17p) ≥20%, and not reached (72.7-NR) for patients with standard-risk NDMM (P<0.0001). Median PFS was not significantly different between patients with del(17p) <20% and del(17p) ≥20% treated with triplet and quadruplet induction regimens (P=0.33). 2-year OS was 89% (95%CI 76%-100%), 85% (74%-98%), and 95% (93%-98%) for patients with del(17p) <20%, del(17p) ≥20%, and standard-risk (P=0.043).
Conclusion: In the MSK experience, the presence of del(17p) was associated with poorer outcomes compared to standard-risk disease in patients with NDMM treated with both triplet and dara-based quadruplet induction regimens. We did not detect a significant difference in PFS and OS amongst del(17p) patients in our cohort using a cut-point of ≥20% or <20% of cells. These findings suggest that the presence of del(17p) alone portend poor prognosis, regardless of FISH cutoff threshold utilized. Data on TP53 mutation status and longer follow-up outcomes will be presented at the meeting.
Disclosures
Hultcrantz:Amgen, Daiichi Sankyo, GlaxoSmithKline: Research Funding; Curio Science LLC, Intellisphere, Bristol Myer Squibb, GlaxoSmithKline: Honoraria. Hassoun:Celgene, Takeda, and Janssen Pharmaceuticals: Research Funding. Mailankody:Janssen Oncology: Consultancy; Janssen Oncology: Research Funding; MJH Life Sciences: Honoraria; Fate Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Physician Education Resource: Honoraria; Allogene Therapeutics: Research Funding; Optum Oncology: Consultancy; Legend Biotech: Consultancy; OncLive: Honoraria; Takeda Oncology: Research Funding; Caribou Therapeutics: Research Funding. Shah:Plantable: Research Funding; Sabinsa: Research Funding; C4 Therapeutics: Research Funding; M and M Labs: Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board, Research Funding; Janssen: Consultancy, Other: Advisory Board, Research Funding; Sanofi: Other: Advisory Board. Shekarkhand:Genentech: Consultancy. Lahoud:MorphoSys Inc, Kite: Consultancy. Shah:Janssen: Research Funding; Amgen: Research Funding; Beyond Spring: Research Funding; BMS: Research Funding; ArcellX: Other: DSMB. Scordo:Medscape, LLC: Honoraria; CancertNetwork (Intellisphere LLC): Honoraria; Omeros Corporation: Consultancy, Research Funding; Angiocrine Bioscience, Inc.: Research Funding; Amgen, Inc.: Research Funding. Landau:Karyopharm, Pfizer, Juno, Prothena, Caelum Biosiences, Legend Biotech, Takeda, Janssen, Nexcella: Honoraria; Alexion Pharmaceuticals, Takeda, Janssen, Prothena, Protego: Research Funding. Giralt:Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding; Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees. Lesokhin:Pfizer: Honoraria, Research Funding; Bristol Myers Squibb: Research Funding; ArcellX: Consultancy; Janssen: Honoraria, Research Funding. Korde:CCO, OncLive, Intellisphere, Remedy Health: Consultancy; Amgen, Janssen, Epizyme, AbbVie: Research Funding; Janssen: Other: Advisory Board. Usmani:Array Biopharma: Research Funding; Merck: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; EdoPharma: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Moderna: Membership on an entity's Board of Directors or advisory committees; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Tan:Takeda: Research Funding; Sanofi: Honoraria; Janssen: Current Employment, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal